Total EDCTP investment in TB research & development rises to € 228 million
On World TB Day 2020, the COVID-19 pandemic is a stark reminder of the global impact and burden of infectious diseases on individual and public health, society and national economies. Since 1997, the World Health Organisation (WHO) has reported on the global tuberculosis (TB) epidemic. Though there has been much progress in fighting TB, in 2018, an estimated 10.0 million people globally infected with TB bacteria got sick, while 1.5 million people died from TB. An estimated 3 million people remain out of reach, undiagnosed and untreated.
It is time – the theme of this and last year’s World TB Day – to end the TB epidemic. In September 2018, all members of the United Nations committed to this goal. However, the world is not on track to pass the milestones set in the WHO End TB Strategy. Funding is insufficient, including for research and development of much needed medical interventions such as a vaccine to prevent different forms of TB or better diagnostics and therapeutics
“Our message remains clear: TB is a preventable, treatable and curable disease which continues to disproportionately blight the lives of the poor and vulnerable. Moreover, drug-resistant strains of the bacterium present a global threat. Our steadily growing investments in TB R&D portfolio of grants on novel diagnostics, more patient-friendly treatments, and new vaccines – especially targeting vulnerable populations at highest risk of TB – shows our enduring commitment to fight this scourge.”
Dr Michael Makanga, EDCTP Executive Director
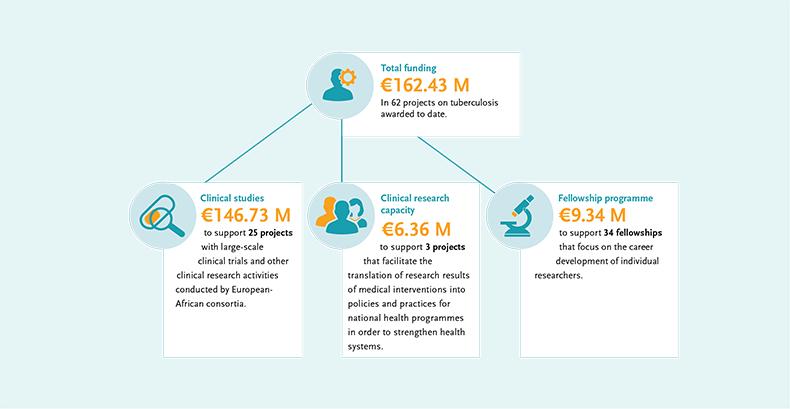
EDCTP investments in TB research
EDCTP is a partnership between, currently, 16 African and 14 European countries strongly supported by the European Union under its Research and Innovation programmes. The first EDCTP programme (2003-2015) committed €65.6 million to research on TB and TB-HIV co-infection. It made a significant contribution to the WHO-recommended TB control strategy, it mobilised European and African funding for TB-related research, and generated important evidence on TB diagnostics and treatment regimens supporting policy changes.
Under its second programme (2014-2024), EDCTP is funding 62 TB research projects, with total funding of €162.4 million to date, across the full array of interventions: diagnostics and case management, treatment and implementation, preventive vaccines. Vulnerable groups are included and co-infections are addressed.
Tuberculosis - one step further
Diagnostics
A rapid triage test for active TB is an urgent priority in the strategy to end TB. To address this challenge, two recently started projects aim to evaluate cheap, easy triage tests for TB that can be used in low-resource settings, with limited lab facilities and few trained personnel.
The TB TRIAGE+ project will evaluate two promising tools for community-based tuberculosis triage testing in hard-to-reach African populations. The main objective of the project is to assess the accuracy, impact and cost-effectiveness of these candidate tools. The tests will be deployed after symptom screening and before on-site confirmation testing with the on-site Xpert MTB/RIF assay. EDCTP awarded a grant of almost €3.2 million.
A first study will evaluate the two tools, i.e. CAD4TB and POC-CRP. CAD4TB is an innovative, digital chest X-ray analysis platform, developed by the Radboud University of Nijmegen, the Netherlands. POC-CRP is a point-of-care test using an acute-phase protein (C-reactive protein) measured in blood indicating systemic inflammation, including active TB. A second study will assess the impact of the triage tools on TB case management and patient outcomes. The study is led by Dr Klaus Reither (Swiss Tropical and Public Health Institute, Basel, Switzerland) with project partners in Belgium, Germany, Lesotho, the Netherlands, South Africa, and Switzerland.
The SeroSelectTB clinical trial is led by Prof. Carol Holm-Hansen (Norwegian Public Health Institute) with partners in Austria, Ethiopia, France, Germany, the Netherlands, South Africa, and Tanzania. The study will evaluate the feasibility, accuracy, and effect of a rapid point-of-care serological triage test for active TB in high burden, HIV-endemic African settings. The trial will take place in health care posts in Ethiopia, South Africa and Tanzania. EDCPT invested almost €3 million.
SeroSelectTB is a ready-to-use unit and does not require laboratory facilities or highly-trained personnel. The test is based on the identification of a combination of antigens that differentiates between latent and active TB. The main objective of the study is to evaluate the performance of the test and determine the extent to which the test reduces diagnostic delay and expedites treatment.
Treatment, including of co-infections
The Click-TB study aims to identify the most promising combinations of TB drugs from a pool of early-stage candidates from new chemical classes. The consortium has adopted a novel trial methodology that will rapidly generate data in order to efficiently identify the most promising combination for a phase III trial. The project is led by Dr David Barros (GSK I&D, Spain) with partners in Germany, Spain and South Africa. EDCTP awarded €6.9 million to the €11 million project.
The EDCTP-funded (€9.6 million) INTENSE TBM study – led by Professor Fabrice Bonnet (University of Bordeaux, France) – is evaluating a revised, more intensive treatment of patients with tuberculous meningitis, including those with HIV infections. The study will assess the impact of this approach on mortality and the likelihood of longer-term disability, generating data on a tailored regimen for those infected with HIV. Consortium partners are in Côte d’Ivoire, France, Madagascar, South Africa, Spain, Switzerland, and Uganda.
The TREATS study will show whether combined HIV and TB interventions targeting entire populations have an impact on the prevalence of TB. It is led by Professor Helen Ayles from the London School of Hygiene & Tropical Medicine, UK, with partners from France, Germany, the Netherlands, South Africa, the United Kingdom and Zambia. EDCTP awarded a grant of €12.9 million to the €26.3 million project.
Vaccines
To achieve the global End TB strategy, safe and effective TB vaccines for all are necessary. Therefore, EDCTP has invested a total of €31.8 million in grants to three TB vaccine studies:
- MTBVAC-Newborns aims to establish whether a weakened form of the TB bacterium is suitable for large-scale trials, potentially offering a better alternative to BCG.
- POR TB evaluates a novel strategy for protecting against TB by preventing latent infections to recur;
- priME will determine whether a promising alternative to BCG is safe and effective for use in newborn infants.
These large innovative clinical studies will be supported to enhance their collaboration with each other and the TB vaccine research community. To that end, EDCTP awarded a contract to the TB Vaccine Initiative (TBVI) to facilitate coordination and collaboration among these consortia. The TBVI project is to develop, establish and make publicly available a set of guidance documents and web-based tools, as well as organise one or more stakeholder meeting(s) to promote collaboration with the TB vaccine research community in Africa and Europe. TBVI already organised a first stakeholder meeting for EDCTP-funded projects at the annual TBVI meeting in January 2020 to introduce various initiatives, including establishing a directory of clinical trial sites in sub-Saharan Africa suitable to perform TB vaccine studies.
The Amsterdam Institute for Global Health and Development (AIGHD) received financial support for the development of a Global Roadmap for research and development of TB vaccines. The roadmap is expected to define what clinical development, key capacities, and policy and access measures are needed to deliver TB vaccines and ensure their public health benefit for all. It will also consider issues of licensure, manufacturing and delivery. The aim is to provide global stakeholders such as researchers, funders, industry, regulatory and policy decision-makers with key actionable priorities. A consensus meeting was held on 3-4 March 2020 in Amsterdam bringing together a diverse group of stakeholders. The primary deliverable of this meeting is a draft Roadmap for public consultation, with the final version ready by August 2020 and WHO endorsement expected later in the year.
Projects of EDCTP member countries
The EDCTP2 programme encompasses projects that are initiated and funded by its member countries independently. These so-called Participating States’ Initiated Activities (PSIAs) are considered a contribution to the programme if they are within the scope of the EDCTP programme and contribute to its objectives. Both African and European member countries conduct projects that qualify as PSIAs.
Several African member countries have submitted projects focused on TB. South Africa, for example, has funded the preparation of clinical trial sites for evaluating host-directed TB therapies. Uganda contributed to studies evaluating the impact of new TB diagnostics on patient health outcomes.
The United Kingdom funded studies led by the MRC Unit in The Gambia to develop and patent a novel diagnostic biosignature. Moreover, the studies led to a doubling of the notification of childhood TB cases in The Gambia and contributed to a new case classification for research in childhood TB. Denmark’s Statens Serum Institut developed a novel Mycobacterium tuberculosis test to detect latent TB infection, the C-TB skin test. And the Spanish Agency for International Development Cooperation (AECID) funded research on TB incidence and epidemiology in Mozambican children.
More information
- WHO Global Tuberculosis Report 2019
- WHO Global Tuberculosis Report 2019 Executive Summary
- TB Triage+ project website
- Intense TBM project website
- Treats project website
