COAST-Nutrition completes recruitment
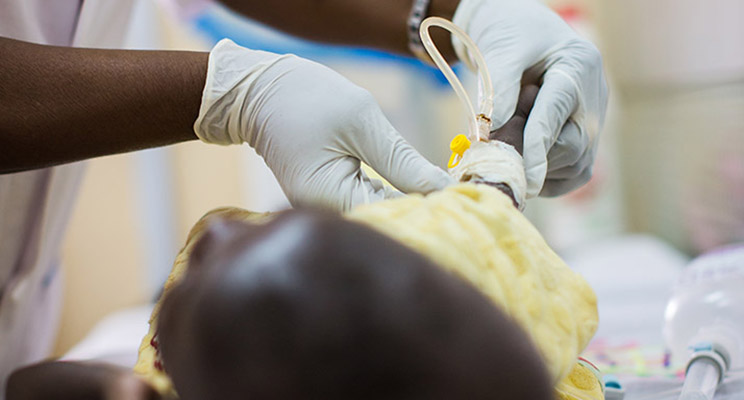
The COAST-Nutrition team is undertaking a phase II clinical trial in children aged 6 months to 12 years hospitalised with suspected severe pneumonia. The team aims to establish whether supplementary feeds with ‘ready to use therapeutic feeds (RUTF)’ given in addition to usual diet for 56-days (experimental) improves outcomes at 90-days compared to usual diet alone (control). The study is being conducted in four sites in two countries (Uganda and Kenya) and has completed the enrolment of 807 children in April 2022. It is expected that the follow-up period of the last patient will be completed by October 2022.
