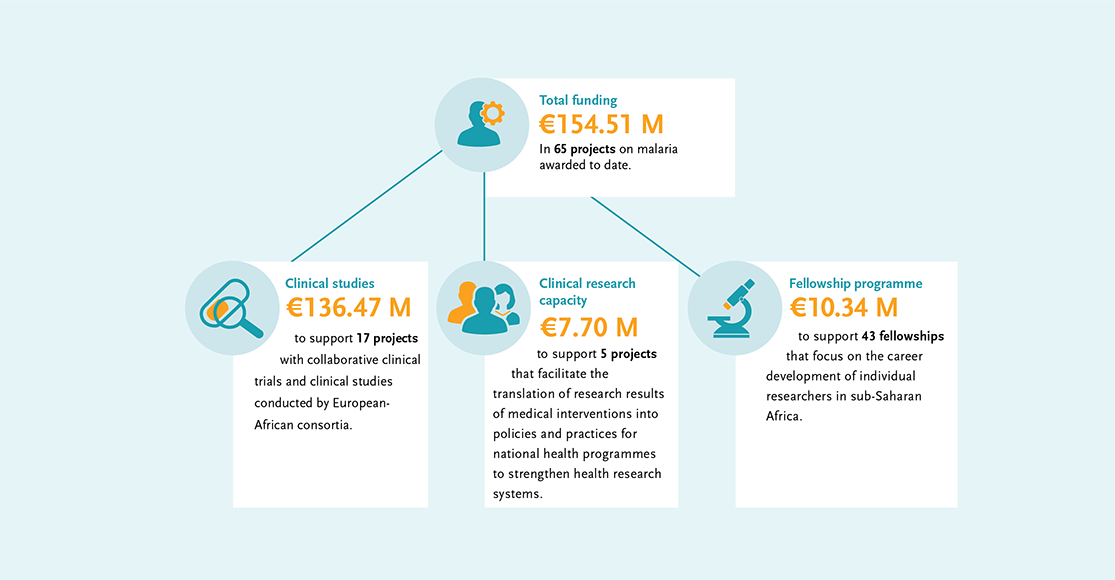
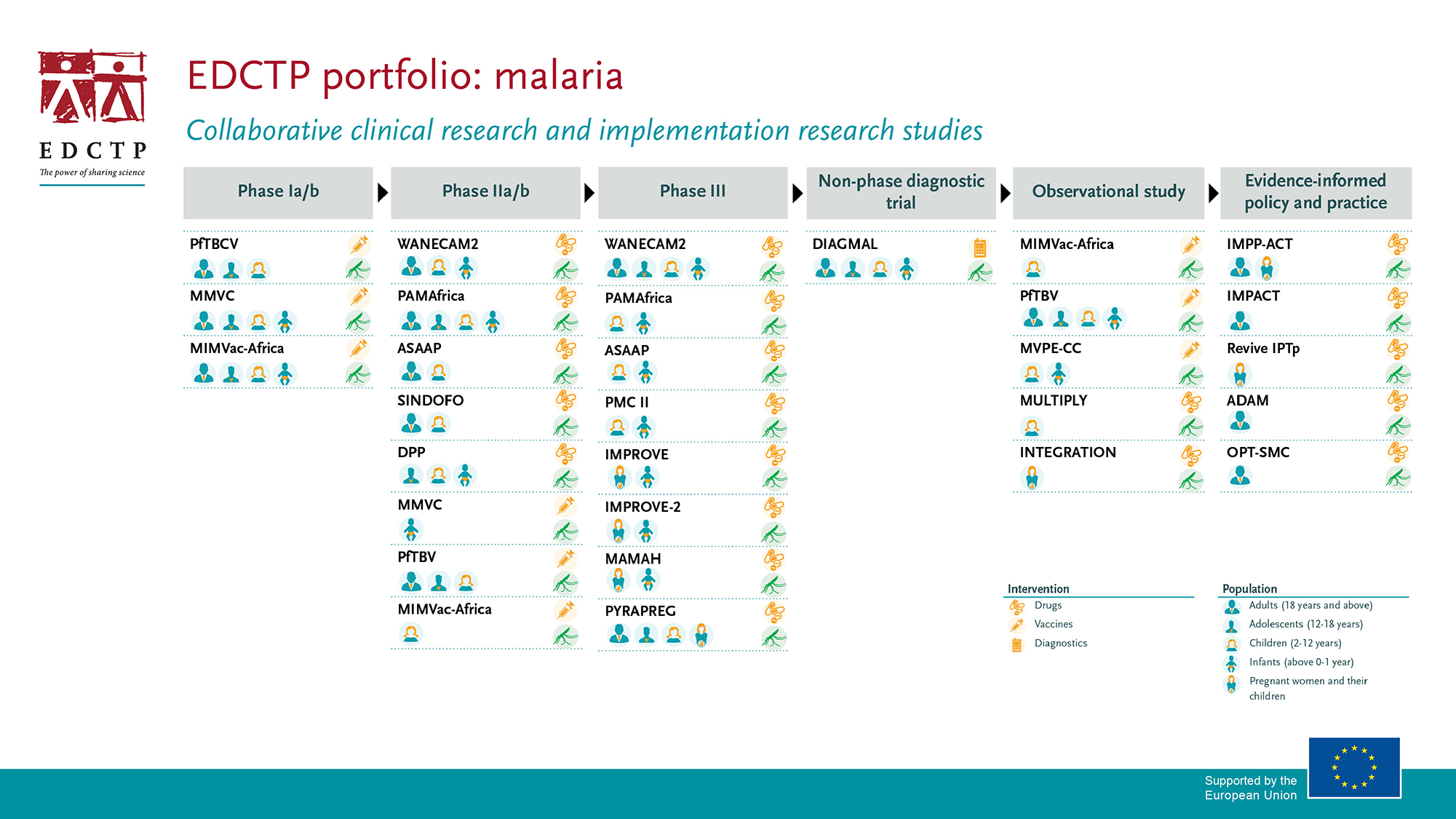
EDCTP’s malaria portfolio has a strong focus on prevention and treatment of malaria in vulnerable populations such as children and pregnant women, and malaria patients with co-infections, accelerating the development of new combination drug treatments, and advancing candidate malaria vaccines.
Our malaria research funding strategy prioritises the following areas:
- Evaluation of new drugs and drug combinations, with a particular focus on children and pregnant women and uncomplicated malaria. Since the majority of individuals living in malaria-endemic areas are exposed to multiple infections, it is increasingly important to understand interactions between antimalarials and drugs used in the treatment of other diseases such as HIV, TB and NIDs.
- Field testing of diagnostics to identify infection and resistance mutations.
- Evaluation of new malaria vaccines.
- Evaluation of the effectiveness of intervention strategies for drugs, vaccines and diagnostics, in the context of malaria elimination.