A growing malaria R&D portfolio: grants and calls 2014-2018
The fight against malaria requires a concerted effort of many and various partners. EDCTP wholeheartedly supports the fight for a world free of malaria that goes back more than 100 years. To end malaria much effort through a combination of approaches is still needed including better medical interventions. As a research funder, EDCTP prioritises R&D on novel and improved drugs and drug combinations, studies in high-risk populations such as children and pregnant women, and malaria patients with co-infections. For prevention, it aims to invest in chemo-prevention for high-risk populations and development of second generation vaccines against both Plasmodium falciparum and vivax malaria.
“We plan to bolster our malaria R&D investment this year by launching two large calls for proposals covering malaria vaccine and novel antimalarial drug clinical development. The novelty in our approach this time is to support strategic clinical research projects that - as part of bigger malaria portfolios - will make a significant difference in the development of new medical interventions for prevention and treatment.”
Dr Michel Makanga, EDCTP Executive Director
Malaria calls for proposals 2018
The EDCTP malaria research funding strategy prioritises the following areas:
- Evaluation of new drugs and drug combinations, with a particular focus on children and pregnant women and uncomplicated malaria.
- Interactions between antimalarials and drugs used in the treatment of other diseases such as HIV, TB and NIDs.
- Field testing of diagnostics to identify infection and resistance mutations.
- Evaluation of new malaria vaccines.
- Evaluation of the effectiveness of intervention strategies for drugs, vaccines and diagnostics, in the context of malaria elimination.
In the second quarter of 2018, EDCTP will launch eleven calls for proposals, most of which open to malaria research proposals. However, two major calls are specifically dedicated to malaria research:
- Strategic action for overcoming drug resistance in malaria. The purpose of this call is to support a large-scale strategic action (clinical research activities) that is part of a bigger portfolio of clinical trials with the capacity to develop new and divers antimalarial drugs against Plasmodium falciparum and Plasmodium vivax, including combination therapies that may be used for treatment or chemoprevention of malaria in sub-Saharan Africa.
- Strategic action for the comparison, selecting and development of malaria vaccine candidates. The purpose of this call is to support a large-scale strategic action (clinical research activities) that is part of a bigger portfolio of clinical trials with the capacity to compare and select the most promising malaria vaccine candidates, and manage their progress through clinical development.
EDCTP malaria portfolio 2014-2017
Under the first EDCTP programme, malaria research received a total funding of € 50.2 million for 42 projects. Since the start of the second EDCTP programme in 2014, 13 projects in malaria research have been funded so far, to a total amount of approximately € 31.9 million.
Two major clinical research projects finalised their grant agreements recently:
- The MAMAH project is coordinated from the Instituto de Salud Global (ISGlobal) in Barcelona, Spain by Professor Clara Menéndez Santos. (ISGlobal webpage) with an EDCTP grant of €2,98 million. It’s title is ‘Improving maternal and infant health by reducing malaria risks in African women: evaluation of the safety and efficacy of dihydroartemisinin-piperaquine for intermittent preventive treatment of malaria in HIV-infected pregnant women’.
- The MMVC project is coordinated from the University of Oxford by Professor Adrian V.S. Hill with an EDCTP grant of €15 million: ‘Multi-stage malaria vaccine consortium: field efficacy testing of a multi-stage malaria vaccine.’
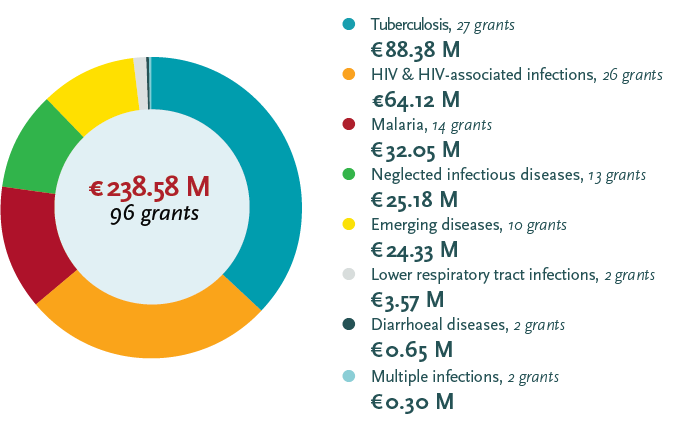
EDCTP grant portfolio 2014-2017 by disease
EDCTP malaria projects by intervention
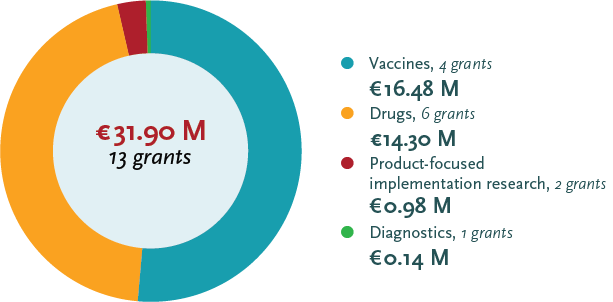
Note on the portfolio 2014-2017: One grant on malaria research capacity development is disease specific but not intervention specific, hence the difference in number of grants between the two graphs. A further € 20 million for 29 grants was awarded to projects on non-disease specific topics such as ethics and regulatory support, networking and fellowship grants. All figures include both estimated and actual value of grants.
EDCTP at MIM 2018
As part of its sponsoring of and contribution to the successful MIM2018 conference on malaria in Dakar, Senegal from 15-20 April 2018, EDCTP organised a symposium on malaria in pregnancy programmes. The presentations focussed on the ‘challenges and priorities in antimalarial drug development for African pregnant women’ the practical challenges, priorities, and lessons learned by researchers conducting clinical trials in pregnant women in resource-limited settings (past and present). The presentations discussed previous and current studies on malaria in pregnancy supported by EDCTP.
One of the projects featured in the symposium was the IMPROVE project coordinated from the Liverpool School of Tropical Medicine (United Kingdom) by Professor Feiko ter Kuile and funded in 2016 with an EDCTP grant of €7,39 million.
The project studies ‘Intermittent preventive treatment in pregnancy (IPTp) with dihydroartemisinin-piperaquine (DP) and azithromycin (AZ) for malaria, sexually transmitted and reproductive tract infections in pregnancy in high sulphadoxine-pyrimethamine (SP) resistance areas in Kenya, Malawi, and Tanzania.’
The World Health Organization (WHO) has recommended further research in IPTp during pregnancy in both HIV-uninfected and HIV-infected women, as the current WHO policy – though still highly cost-effective in most endemic African countries – is compromised by the risk of parasite resistance to SP and it is contraindicated in HIV-positive pregnant women receiving cotrimoxazole prophylaxis (CTXp), leaving the more vulnerable women the less protected.
The EDCTP-funded IMPROVE project has a closely aligned counterpart in a study with HIV-infected pregnant women, IMPROVE-2: ‘IPTp with dihydroartemisinin-piperaquine and azithromycin for malaria, sexually transmitted and reproductive tract infections in HIV-infected pregnancy in Kenya and Malawi: a multicentre 3-arm placebo-controlled trial’. This study is coordinated by Professor Ter Kuile as well and funded with a grant of £2.7 million by the United Kingdom Joint Global Health Trials, a collaboration of the Department for International Development, the Medical Research Council, the National Institute for Health Research and the Wellcome Trust.
The projects aim to provide WHO with definitive evidence to determine whether IPTp with DP (with or without AZ), is a viable alternative to the IPTp with SP. A positive result would improve the outcome of pregnancies in areas of the world with moderate to high malaria transmission and high parasite resistance to SP.
More information
The World Health Organization WHO celebrates its 70th anniversary and shares on the occasion of World Malaria Day 2018 seven interviews with leaders and advocates in the global malaria response
The series includes the following seven interviews with:
- Dr Kesete Admasu, CEO, RBM Partnership to End Malaria: “United for malaria”
- Dr Pedro Alonso, Director, WHO Global Malaria Programme: “7 decades of malaria”
- Mr Bill Gates, Co-Chair, Bill & Melinda Gates Foundation: “Stepping up for malaria”
- Dr Matshidiso Moeti, WHO Regional Director for Africa: “Beating back malaria in Africa”
- Dr Lynda Ozor, Malaria focal point, WHO Nigeria: “A view from the frontlines”
- Dr Arantxa Roca-Feltrer, Head, Monitoring & Evaluation, Malaria Consortium: “From bites to bytes”
- Dr Neena Valecha, Director, National Institute of Malaria Research, India: “Behind the microscope”
- WHO – 2017 World Malaria Report
- WHO – WHO Global Malaria Programme at the 7th MIM Pan African Malaria Conference
- Roll Back Malaria Partnership – World Malaria Day 2018
- Roll Back Malaria Partnership – Commonwealth leaders commit to halve malaria across the Commonwealth by 2023
- Bill & Melinda Gates Foundation – Malaria strategy
- EDCTP Strategic Business Plan 2014-2024
- EDCTP Strategic Research Agenda 2017
Please note that the two up-coming calls for proposals were mentioned pending formal approval of the EDCTP work plan 2018 by the European Commission.
